Products
Your all-inclusive solution
All Products
Download our catalog
Get our Catalog
Applications
Pioneering the exploration of uncharted areas of Life Sciences, our innovative cameras are the solution to the most complex detection challenges of the scientific community involved in ultra-low light imaging.
Multimodal imaging allows parallel acquisitions in a single exposure as it applies different probe or modalities simultaneously. Becoming a standard in the pre-clinical industry, it operates by injecting molecular markers detectable by multiple modalities like positron emission tomography or optical tomography. For example, one can resort to a slightly radioactive and fluorescent probe, in which emissions are then detected by a camera and another system.
Each of the probe’s modalities has a different concentration interval in which it remains detectable and safe. Different modalities may have overlapping sensitivity ranges; others may be harmful at high enough concentrations, but concentrations that would lead to visible fluorescence. Finally, some modalities may hinder fluorescence in increasing concentrations. In all cases, the probe’s emission is faint, thus subject to noise contamination.
While using the same EMCCD sensors as all of its competitors, Nüvü Camēras offers “very attractive characteristics for ultra-low light and ultra-sensitive optical biomedical imaging with the best SNR in low light applications” (Bérubé-Lauzière, 2013). In the case of multimodal fluorescence imaging, it effectively answers the issue of the diminished probe’s emission at low concentration. Furthermore, with pixel rates reaching up to 20MHz, Nüvü™ EMCCD cameras support 360° live optical tomography acquisitions.
Y. Bérubé-Lauzière’s team at the University of Sherbrooke presented results of in vitro and in vivo characterization of zinc- and copper-trapped phthalocyanines, multimodal biomarkers, and determined the detection limits of such with a Nüvü camera. The results of his research conducted with his team at the University of Sherbrooke are shown in the conference proceeding below.
Raman spectroscopy studies vibrational, optical, and electronic properties of materials in a non-destructive manner. In particular, the technique helps to identify and label molecules by assessing their symmetry and chemical bonds. With Raman imaging, it is possible to depict an object of interest, plus obtain a complete Raman spectrum per pixel.
Raman scattering generates extremely weak signals and thus requires long exposure periods to perform suitable molecular analysis. Refining the spectral resolution leads to increasingly longer acquisition periods as light intensities reaching the camera further decrease.
With Nüvü Camēras’s unrivalled sensitivity, consequence of a lower overall background noise, Raman spectroscopy and imaging can be accelerated up to ten times without resorting to more complex methods such as coherent anti-Stokes (CARS) or surface-enhanced (SERS) Raman spectroscopy. The spectral or spatial scanning benefit from great savings in acquisition time.
F. Thouin (University of Montreal) showed the efficiency of Nüvü™’s EMCCD camera for performing ultrafast Raman spectroscopy measurements. Their results are presented in the poster available below.
Meanwhile, Prof. Frédéric Leblond’s team (University of Montreal) demonstrated a handheld wide-field Raman spectroscopy probe for projected use in neurosurgery.
Fluorescence-guided surgery lies on the property of certain agents to selectively accumulate in particular tissues, thus better revealing the boundaries between healthy and unhealthy cells. Improving this surgical technique will ultimately lead to better tissue resection for multiple diseases such as brain and ovarian cancers. The complete removal of malignant cells will increase a patient’s remission and prognostic.
Fluorescent marker in vivo imaging results in a faint, low incoming signal. To efficiently guide surgeons in the operating room, long exposure times are required for enough photons to accumulate in the sensor wells. Longer integration times lead to higher dark current levels but also increase the length of the operation procedures, which may alter a patient’s life expectancy during delicate surgery.
Nüvü Camēras’ top-of-the-line EMCCD controller effectively cuts down clock-induced charges associated with fast acquisitions. Besides, it supports EM gains up to 5000, rendering readout noise negligible. As these factors allow superior SNRs, they make accurate real-time imaging possible despite the fluorescent marker low incoming signal.
A multidisciplinary team has validated that a spectroscopic EMCCD-based imaging system integrating Nüvü™’s HNü 512 camera surpasses the current sCMOS-based imaging system for protoporphyrin IX fluorescence-guided brain resection. Thorough results are presented in the article below.
A particularly promising biomedical research tool is single-molecule imaging, which detects biomarker emissions with increased resolutions, reaching the molecular scale. However, such a method strongly depends on the stimulating laser intensity that triggers the fluorophore emission.
Fluorescence-based imaging is hindered by marker photobleaching, which, in turn, depends on the stimulating laser intensity as well as measurement duration. Optimizing exposure time and trigger intensity is key for achieving high-quality single-molecule imaging.
Nüvü™ offers EMCCD cameras that support greater frame rates and temporal resolutions as well as the lowest overall background level on the market. Nüvü Camēras technology elegantly resolves the photobleaching issue inherent to single-molecule imaging and allows the user to decrease the stimulating laser intensity. The result: Improved fluorophore lifetime and superior image quality at the same time.
The Institute for Research in Immunology and Cancer (IRIC), along with the University of Montreal’s Department of Pathology and Cell Biology, confirm the increased performances of a single-molecule imaging microscopy system using Nüvü™’s EMCCD in photon-counting mode. Their poster is presented below.
Paul De Koninck from the Centre de recherche de l’Institut en santé mentale de Québec demonstrates the ability to detect and track 4 subtypes of receptors at synapses. Even if the number of photons detected in a single QD PSF was on the order of 100 to 150 photons, thereby limiting the localization error usually associated with these optical techniques, the low background signal provided by the Nüvü’s camera allowed for subpixel accuracy.
Bioluminescent reagents are often used in biological assays, such as sanitary evaluations, to assess and quantify the presence of a particular molecule. A prime example is adenosine triphosphate (ATP) detection in biocompatibility and cytotoxicity tests. In such assays, a combination of luciferin and luciferase produces a light-emitting compound in the presence of ATP. The bioluminescent signal varies linearly if ATP concentrations are inferior to those of the reagent pair. As such, calibration curves can be obtained to quantify precisely ATP amounts in biological samples.
Typical detection methods inhibit ATP quantification lower than the picomole scale. Indeed, commercially available cameras do not reach the required sensitivity to observe smaller luciferin+luciferase concentrations.
Nüvü cameras enable better ATP detection as a result of their controller and packaging technology that significantly lower the overall sensor noise. Nüvü Camēras photon counting capabilities further push detection limits, pushing them down to the femtomole scale.
Presented below are three images of the same microplate containing solutions of various ATP concentrations, ranging from 5 to 156 femtomoles and studied in triplicates. Each image was acquired with Nüvü™ EM N2 1024 camera.
30 seconds total exposure time – single frame acquisition and conventional mode at 100 kHz at -85ºC
Figure 1: Single 30-seconds acquisition in Conventional (CCD) mode. The image displays the expected performance of a top-of-the-line CCD camera or even a high-end sCMOS. Without the electron multiplication to boost the bioluminescent signal, ATP solutions are barely noticeable.
5 seconds total exposure time – summation of 5 single frames, each acquired over 1 second, in EM mode at 10MHz with an EM gain of 5000 at -85ºC.
Figure 2: Superposition of five 1-second EM acquisition. With a lower noise floor due to the electron multiplication process, all six ATP concentrations are detectable. SNR values vary from 1.9 to 14.1 (or, equivalently, from 2.8 to 11.5 dB).
5 seconds total exposure time – summation of 5 single frames, each acquired over 1 second, in Photon Counting EM mode at 10MHz with an EM gain of 5000 at -85ºC
Figure 3: Superposition of five 1-second EM acquisition in photon-counting mode. Suppressing ENF, photon counting pushes the SNR, which ranges between 6.6 and 51.3 (8.2 to 17.1 dB). ATP quantities were as low as 5 femtomoles are detected with strong contrast. Images courtesy of the Université de Sherbrooke Hospital Centre.
Prof. Ewa Goldys and her team at The University of New South Wales are pioneers in label-free bio-imaging & tissue characterization. With Nüvü’s HNü 1024, they have successfully investigated the impact of ageing on stem cells using a multispectral analysis of the fluorescence of its different chemical components.
Förster and Bioluminescence Resonance Energy Transfer (respectively FRET and BRET) are common techniques to probe intermolecular reaction kinetics in biology and chemistry. Such events occur on the nanoscopic scale between molecules and hence produce little light.
FRET and BRET imaging share similar drawbacks, namely photobleaching – the photochemical alteration of an emitting molecule due to the trigger light source intensity, applicable for FRET measurements only – and the specimens’ low signals. Such obstacles interfere with data acquisition.
Fortunately, both issues are addressed by Nüvü™ EMCCD cameras, which support shorter exposure times, higher frame rates and offer greater sensitivity, thus better SNR. Furthermore, a user may decrease the laser intensity while performing FRET imaging, hence minimizing photobleaching effects, due to the camera’s superior sensitivity.
The following article presents the work of Prof. Michel Bouvier’s group at the Institute for Research in Immunology and Cancer (IRIC). This research showcases the power of Nüvü Cameras for protein interaction studies with BRET.
Despite the biomarkers’ non-invasive quality, making them appealing for diagnostic and research purposes, small animal imaging suffers from the poor light intensities emitted by these molecules. Worse, a significant portion of their emissions is lost prior to detection through absorption and scattering within the specimens’ tissues, resulting in an extremely weak incoming signal.
Nüvü™ research cameras are ideal for small animal imaging thanks to their unmatched sensitivity in ultra low light conditions because of high EM gains and the greatest SNR among all EMCCD cameras on the market. Furthermore, Nüvü™ EMCCD cameras not only allow the detection of substantially more elusive photons but also reach shorter acquisition speeds while preserving their sensitivity, all to create superior quality images.
Novel studies conducted by F. Lesage’s research group from the Montreal Heart Institute, in collaboration with the Institute of Biomedical Engineering at École Polytechnique de Montréal, demonstrate the potential of Nüvü™ EMCCD cameras for small animal fluorescence imaging. The group presented Ultrasound-guided fluorescence tomography at the 2012 Photonics North meeting where they describe a “hybrid-model imaging system combining fluorescence and ultrasound”.
The figures below illustrate the efficacy of the hybrid fluorescence-ultrasound small animal imaging system in mice. CCy5.5 fluorophores were injected intravenously to a concentration of 0.1 mg/L. The colored zone shows the scanned area acquired by superposing fluorescence images to controls.
Results from another hybrid system applying fluorescence tomography (FMT) and magnetic resonance imaging (MRI) were published by F. Lesage research group in the May 2014 issue of Biomedical Optics Express.
When traveling faster than light in a particular medium, high-energy charged particles, namely electrons and positrons, emit what is known as Cerenkov radiation. Such radiation, commonly measured in high-energy physics experiments, also reveals itself as a promising biomedical imaging tool, whether for biomedical studies or cancer detection.
Injecting a radioactive dye in biological tissues inevitably produces Cerenkov radiation as the dye decays. However, the emission is nearly imperceptible and gets easily absorbed by the tissues. Increasingly sensitive cameras and longer integrations are thus required to detect the low-level Cerenkov emission ranging in the visible and near-infrared spectra.
Nüvü™ EMCCD cameras, such as the HNü series, are perfectly suited for Cerenkov luminescence imaging. With higher quantum efficiency across the visible spectrum and the near-infrared, and ultra-low noise even at high EM gains, Nüvü cameras can detect weak Cerenkov signals in shorter exposure times. Better, photon counting can provide the ultimate precision required for this forefront imaging technique.
Multiple biomedical research fields can now benefit from the most sensitive camera on the market. Observing individual photons at high frame rates with an astoundingly high SNR is now possible with Nüvü™ EMCCD cameras. With a background noise below 0.001 ē/pix/frame as well as an EM peaking at 5000, these cameras can be applied to several biomedical research fields to study phenomena that have never been examined with such precision, sensitivity and reliability.
An EMCCD background noise results from the combination of dark current and clock-induced charges. The readout noise, the excess noise factor, and improper charge transfer also influence the background noise. Nüvü Camēras state-of-the-art and patented technology elegantly overcomes these obstacles. Its lower noise allows signal amplification up to 5000 times via electron multiplication before digitization, while typical EMCCDs are limited to an EM gain of 1000 due to lack of efficiency. Combine with the multitude of other noise-countering methods made available with CCCP technology. Additionally, Nüvü Camēras advance technology sets new standards using creative and innovative engineering to physically reduce the system’s noise without a single computer-based filtering algorithm.
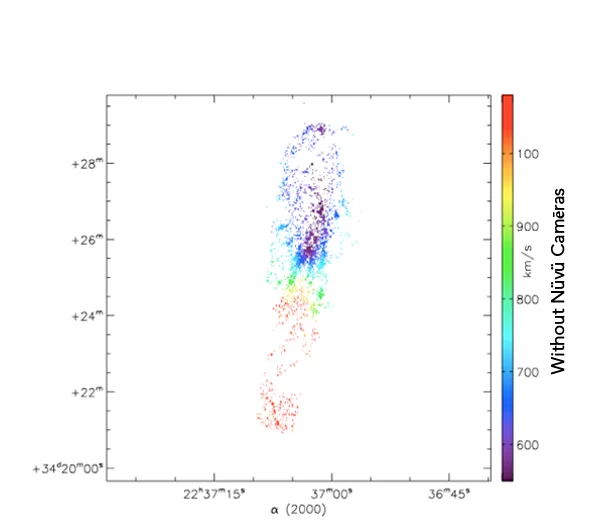
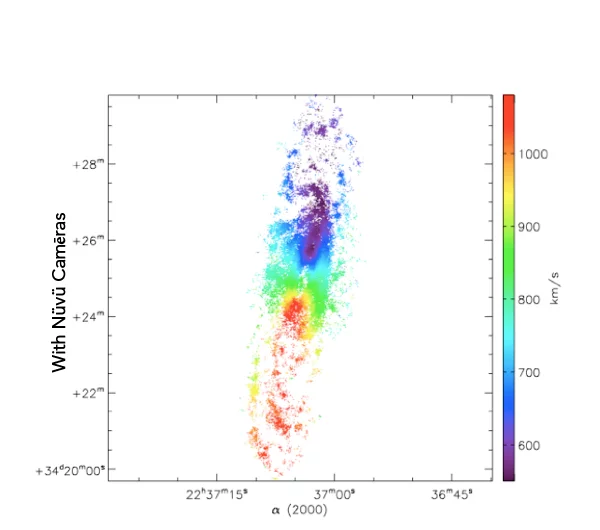
What you see is the exact same galaxie imaged with and without Nüvü Camēras’s imaging technology. Now imagine that each star is a cancer cell, which technology would you prefer?
Spatial innovations have always been at the forefront of new technologies. This is the reason why Nüvü’s technology, although initially designed for Astronomy, is now pushing boundaries of the observable in life science.
Any questions about EMCCD or low light imaging? Nüvü Camēras experts can provide advices on your low light imaging options.